Napkin Math: Feasibility of Human Activities Causing Climate Change
A Review of the Numbers
I find it can be useful to revisit viewpoints that I hold, especially ones around areas that are politically controversial. It can be too easy sometimes to say "Oh, that information doesn't support my current opinion, so I'll disregard it" especially in an area that has a lot of misinformation out there. As an example, a claim that "Of all CO2 emitted into the atmosphere each year, 95-97% is from non-human sources". Which may be approximately true. The implication, though, is "therefore the amount emitted by humans is small, therefore it does not have much/any effect".
So I decided to do a napkin-math, first-principles check to see if the amount of CO2 emitted by humans was capable of causing the observed increase in atmospheric CO2 levels.
Let's start with our base numbers. Pulling from an old IPCC report (archive), natural CO2 emissions from all sources (photosynthesis, vulcanism, etc) amount to 730 gigatonnes(gt). Not the article gives values in petagrams, but 1 petagram == 1gt == 1 million billion grams. These numbers are part of our biosphere and aren't likely to have changed significantly in the time since that report was published. A more recent report(archive) puts human-caused emissions a bit above 30gt. Other values can be found for these values from different sources, but they're all approximately similar, within about 5%.
Checking the above claim: 30gt from humans divided by (730gt natural + 30gt from humans) is 0.0395, or just under 4%, leaving 96% from natural sources. That's squarely in the 95-97% range mentioned above, so that claim checks out.
How much CO2 is being added to the atmosphere each year? Here's a chart from the Scripps Institution of Oceanography, the well-known Keeling Curve:
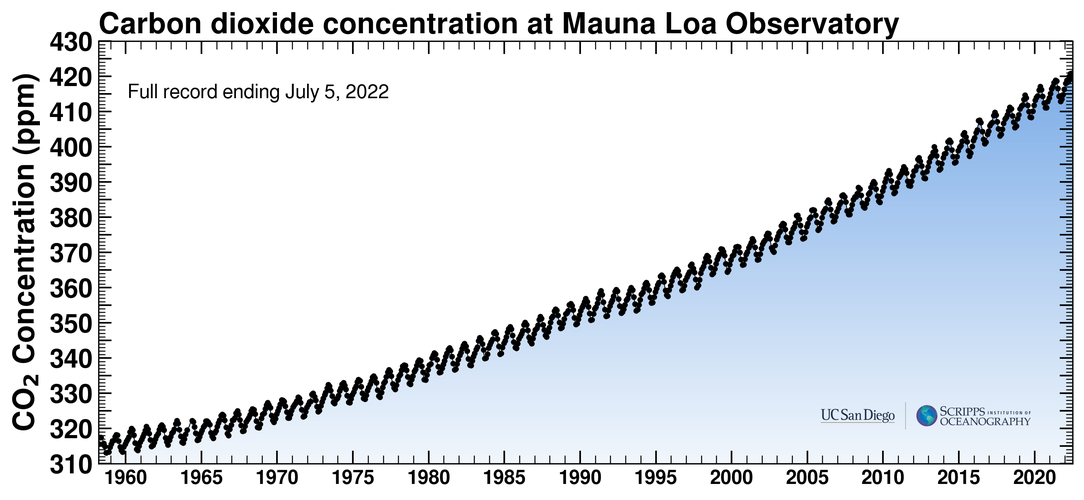
Looking at data from the Mauna Loa observatory(archive), the year for our 30gt estimate above (2014) had a high CO2 level of 401.98ppm, in May of that year. The highest level recorded the previous year, 2013, was 400.06ppm, which is an increase of 1.92ppm.
How much CO2 is 1.92ppm? Well, according to the Encyclopedia Brittanica(archive), the Earth's atmosphere weighs 5.5 quadrillion tons, which is 5.5 million gigatons. This means that a chunk of atmosphere representing on millionth of the total, or 1ppm, weighs 5.5 gigatons.
However, "parts per million" refers to the counts of the actual air molecules, not just weight. If you have a box of air with one million molecules in it, the weight per molecule is going to be an average of the weight of all molecules in the air. Air is mostly nitrogen, and a molecule of nitrogen, N2, is significantly lighter than one molecule of CO2. This means that compared to the 5.5gt figure above, we will need a greater weight of CO2 to add one ppm of CO2 to the atmosphere.
How much heavier is CO2 than air? The way this is measured is in grams per mole, where a mole is a quantity of molecules defined by Avogadro's number, 6.022 * 10^23. This isn't easy to visualize, but a good rule of thumb is that one mole of a solid is approximately the size of your thumb. This weight measurement represents gathering up 6.022*10^23 molecules of what we're trying to measure, and weighing that group of molecules.
The molecular weight of dry air is 28.97 g/mol (driven mostly by N2, which is 28.02 g/mol), while CO2 is 44.01 g/mol.
5.5 gigatons / 28.97 g/mol = 0.1898 petamoles. This is equivalent to 1.143 * 10^38 molecules, one-millionth of the number of molecules in the atmosphere.
We want to know how much CO2 it would take to increase the atmospheric concentration by the 1.92ppm we observed above, and how that compares to actual CO2 emitted by people. 0.1898 petamoles * 44.01 g/mol = 8.355 gt CO2, which is the amount of CO2 we would need to add to the atmosphere to increase the CO2 concentration by 1 ppm. 8.355 * 1.92ppm increase = 16.04 gt CO2 net added to the atmosphere between 2013 and 2014.
So according to our data sources, while it is true that human CO2 emissions are a small fraction of the CO2 emitted each year, the amount of CO2 that annually accumulates in the atmosphere is smaller still. While the math above, in and of itself, does not prove that humans are themselves responsible for the accumulation, it does indicate that it is within our power as a species to reverse the accumulation of CO2 in the atmosphere simply by reducing our own emissions. Regardless of whether or not a person believes that the CO2 accumulation in the atmosphere is caused by humans, it is clear that CO2 levels are increasing, and thanks to the greenhouse effect, that is a problem. And the above shows that we can indeed do something about it.